Water Electrolysis H2 Production
Rahul Subedi
rsub9847877058@gmail.com
Theory
Electrolysis of water is the decomposition of water into oxygen and hydrogen gas due to the passage of electricity. In 1800 Alessandro Volta invented the voltaic pile, and a few weeks later English scientist Willian Nicholson and Anthony Carlisle used it for the electrolysis of water. Zenobe Gramme invented the Gramme machine in 1869 electrolysis of water became a cheap method for the production of hydrogen. A method of industrial synthesis of hydrogen and oxygen through electrolysis was developed by Dmitry Lachinov in 1888.
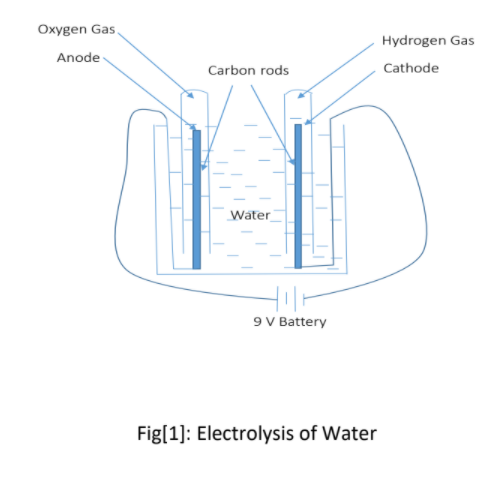
A DC electrical power source is connected to two electrodes, or two plates (typically made from some inert metal) which are placed in the water. Hydrogen will appear at the cathode (where electrons enter the water) and oxygen will appear at the anode. Assuming ideal faradaic efficiency, the amount of hydrogen generated is twice the amount of oxygen and both are proportional to the total electric charge conducted by the solution. Electrolysis of pure water requires excess energy in the form of overpotential to overcome various activation barriers. Without the excess energy, the electrolysis of pure water occurs very slowly or not at all. This is in part due to the limited self-ionization of water. Pure water has an electrical conductivity about one-millionth that of seawater. Many electrolytic cells may also lack the requisite electrocatalysts. The efficiency of electrolysis is increased through the addition of an electrolyte (such as a salt, an acid or a base) and the use of electrocatalysts.
Apparatus Used
Two small bottles
9 V Battery
Copper Wire
Carbon rod (extracted from the battery)
Small Basin Bucket
Sodium Hydroxide (NaOH)
Procedure Used
First of all, water was taken in a small basin bucket. Sodium Hydroxide (NaOH) which was used as electrolyte dissolved in a water. Two small bottle was taken and it was calibrated using 10 ml lid of a bottle with a mark so as to know the volume of gas produced. Then these two bottles were filled with water in a small basin water and kept in the water with upside down position. Two carbon rods were connected to positive and negative terminal of 9 V battery respectively with the help of wire. One carbon rod was kept inside one bottle filled with water and another carbon rod in another bottle. Slowly, reaction occurred and bubbles started to form and gas was collected in the small bottles with the downward displacement of water. At anode, positive terminal connected carbon rod oxygen gas was formed and at cathode, negative terminal connected carbon rod hydrogen gas was formed
Reactions:
At Anode:
4OH– O2 + 2H2O + 4e– -0.4V
At Cathode:
4H2O + 4e– 2H2 + 4OH– -0.83V
Overall reaction:
2H2O O2 + 2H2 Eocell = -1.23V
Observation
Bubbles were observed and gas was formed at both bottles with the downward displacement of water. Volume of gas produced at cathode which is hydrogen is double the volume of gas produced at anode which is oxygen was observed. Rate of bubble formation was greater with increasing the electrolyte.
Test
For test, whether produced gas was hydrogen or not, burning stick was exposed to produced gas at the bottle. There must occur pop sound as the hydrogen reacts with oxygen in the air in a small explosion. But pop sound was not occurred which may be due to the production of very small volume of gas.
