Water Electrolysis H2 Production
Prashrit Sapkota
prashritsapkota@gmail.com
The first edition of the “Lecture Series on Hydrogen Technologies” offered by the Green Hydrogen Lab has been a successful conducted. It was a great learning for enthusiastic learner like us in Green Hydrogen Technology. Lecture included technologies related to production, storage and utilization of hydrogen. As part of the course all I did a “Home Project on Hydrogen Production” by electrolysis of water using home appliances. This report consists a simplified detail of production done my me.
Theory
The electrolysis of water is mainly carried out to yield pure hydrogen and oxygen gases. It involves
passing an electric current through the water which results in the decomposition of water into hydrogen
and oxygen. Hydrogen obtained through electrolysis is a clean, renewable and efficient fuel source. In
this experiment sulphuric acid was used. Additional hydrogen ions from acid will be reduced at the
cathode while water will be oxidized at the anode. Half reactions in an acid medium are;
At cathode: 2H+ + e– → H2 E° = +0.0 V
At anode: 2H2O → O2(g) + 4H+ + 4e– E° = +1.23 V
Net reaction is written as 2H2O → O2(g) + 2H2 E° = -1.23 V
The electrolysis takes place at a much lower potential than pure water (2.4V).
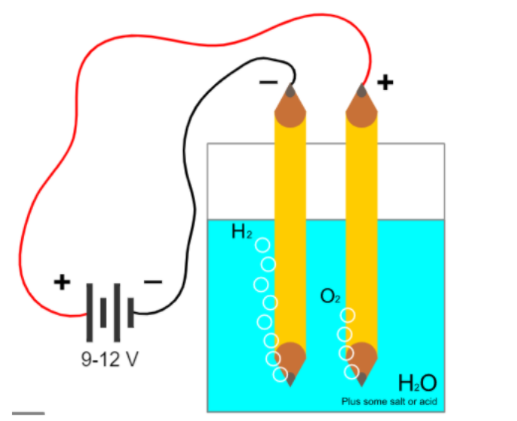
| Materials Required | Physics Behind it |
| Two Beakers | Used as Voltameter for doing Electrolysis |
| Measuring Cylinders | To measure the amount of hydrogen produced by downward displacement |
| Two pencils | Pencil Lead made up of graphite can be used as carbonated electrode |
| Two wires | Wires to connect the electrode to the power supply for electrolysis |
| Dilute Sulphuric Acid | Used as electrolyte to add Hydrogen ions in water and enhance electrolysis |
| 9V DC Power Source | To break cell potential of water more than 2.4V power source is needed. We have used electrolyte and for factor of safety take power source of 9V. |
Testing
• Electrolysis was done for 5 mins for analysis
• At initial phase bubbles started to form at both electrode
• At the end of 5 min of electrolysis at cathode 3.9 ml of water is displaced and 2 ml is displaced at anode
• This shows that twice of hydrogen is produced than oxygen at anode
• Confirmation of hydrogen is done by combustion and “POP “sound was heard.
Validation
• Validation is of hydrogen and oxygen ratio in water 2:1
• To validate amount of hydrogen we can use faradays first law of electrolysis Mass of Hydrogen deposited at cathode = ZIT
Where Z is a constant known as electrochemical equivalent and is characteristic of a substance deposited and I and T are current and time respectively.
So, from experiment mass deposited in 5 min of electrolysis = 0.000357 gm (Mass of 3.9 ml hydrogen)
From faraday’s law mass to be deposited in 5 min of electrolysis = 1.5*300*1/96485 = 0.0046 gm
Since experiment was done in household conditions and from following data efficiency of electrolysis was nearly 10% which was poor.
Conclusion
From experiment we can conclude that electrolysis can be done at home and main factors affecting electrolysis is electrode chosen, electrolyte, and electric current. The effect of the gas bubble surrounding the electrode on the further electro transfer may decrease the efficiency. From this experiment we can develop the study of alkaline electrolysis too. Where dilute aqueous sodium (or potassium) hydroxide used in the electrolysis provides and movement of hydroxide ions to the anode to form oxygen.
