Water Electrolysis H2 Production

Abhishek Subedi
abhisheksdi96@gmail.com
PROJECT OBJECTIVE
To Demonstrate the Production of H2 Gas Via Electrolysis Using Household Equipment.
MATERIALS REQUIRED
- Container
- Plastic slit
- Spoon x 2 and wires
- Pen shell
- Plastics
- Distilled water
- Charger
THEORY
Electrolysis of water is the process by which water is decomposed into oxygen and hydrogen gas, when electric current is passed through it. Water molecule is decomposed into H+ and OH- ions, when electric current is passed through it. These ions move to oppositely charged electrodes and are liberated as gases.
Chemical reaction:
2H2O ——> 2H2 + O2
Half reactions:
Reduction of Hydrogen: 2H2O + 2e–——> H2 + 2OH–
Oxidation of Oxygen: 2H2O ——> O2 + 4H+ + 4e–

PROCEDURES:
- Plastic container was taken and cleaned.
- Plastic slit was inserted.
- Two spoons were placed in and wires (red= +ve and black= -ve) were connected as
shown in figure. - Two pen shells were placed in as shown in figure for the outlet of gases.
- Plastics were tightened to the external end of pen shells for the collection of gases. Black
tape was used to prevent leakage through spoons inlets. - 700 ml of distilled water was kept in the container and lid was tightened.
- Power supply of 5V 2.1 A was connected and the process was observed.
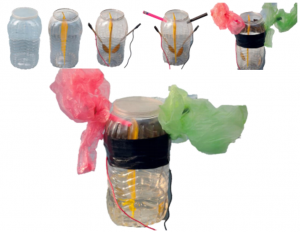
Figure 2 Procedures and Development
OBSERVATIONS, UNDERSTANDINGS AND RECOMMENDATION
| S.N. | Observation | Understandings | Recommendations |
| 1 | It took less than a minute to demonstrate the reactional change in the water. | Because of the use of 5V 2.1 A DC supply and Addition of salt (1 spoon) in distilled water act as a catalyst. | |
| 2 | Significant amount of gas was produced in around 40 minutes of time duration. | Time duration could be less than that but leakage through pen shells and mixing of gas in between the slit is the reason. | Leak proof tapes or adhesives can be used to prevent leakage. Different arrangements can be used for positioning the slit as to stop mixing the gases. |
| 3 | Black yellowish color in the water. | Because of the surface erosion of slit and spoon. | Non-corrosive and non-erosive materials can be used. |
